Our sun protection activities
The various photoprotection test methods supplied by solar products
The evaluation of sun protection is based on three distinct and complementary methods that measure optimal protection against the sun’s UV rays:
- An in vitro method based on transmittance measurement,
- An in vivo method based on a biological response,
- A hybrid method(in vivo/invitro).
Analytical method
In vitro
PRINCIPLE
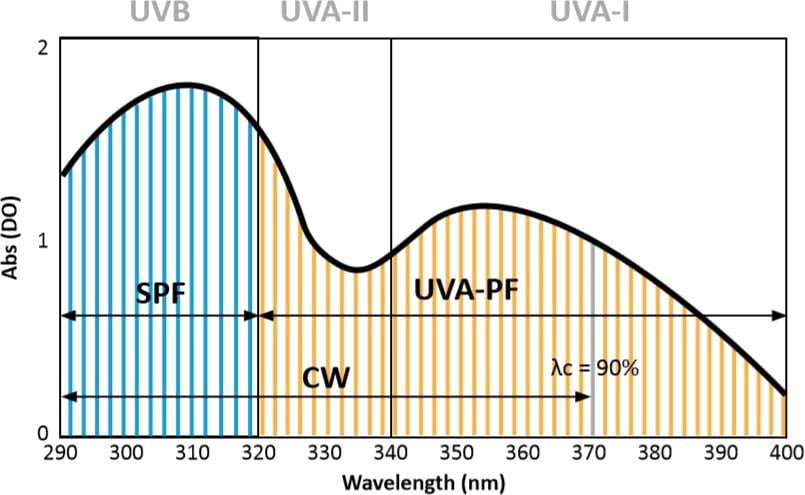
The in vitro method is based on measuring the residual transmission spectrum through a layer of product applied to specific substrates.
This assessment is carried out using a spectrophotometric technique, which quantifies the amount of UV radiation that passes through the product, in order to estimate its effectiveness in blocking UV (UVB and UVA).
Standards
- ISO/DIS 23675: Cosmetics – Sun protection test methods – In vitro determination of sun protection factor (SPF)
- ISO 24443:2021: Cosmetics – In vitro determination of UVA photoprotection
- FDA 2021 : U.S. Food and Drug Administration – Final Administrative Order (OTC000006) – Over-the-Counter Monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use
- Boots Star Rating System 2011: Measurement of UVA:UVB ratios
Benefits
- No ethical problem (non-human model),
- Measuring FPS, FPUVA and Critical Wave Length,
- Optimized repeatability and reproducibility,
- Improvements in terms of time and costs,
- Elimination of technically difficult procedures (e.g. visual reading of DEM),
- A method conducive to continuous improvement.
YOUR STUDIES with WENEOS
- Analyses carried out in-house at our center of expertise.
- In vitro results for a product in less than 7 days.
Hybrid in vivo / in vitro method
NEW | HDRS
PRINCIPLE
The HDRS method proposes a hybrid approach combining in vitro and in vivo tests to assess the Sun Protection Factor (SPF), UVA Protection Factor (UPF) and Critical Wavelength (CW) of sunscreens.
It uses diffuse reflectance spectroscopy (DRS) to measure UVA absorbance (320-400 nm) on the skin of a human volunteer with and without prior application of a sunscreen product.
To obtain a complete UV absorbance spectrum, the in vitro absorbance values are adjusted according to the data obtained via DRS, enabling the in vitro UVB absorbance to be mathematically linked via hybridization to the UVA portion measured by DRS in vivo.
Standard
- ISO/DIS 23698 : Cosmetics – Measurement of the efficacy of sunscreen products by diffuse reflectance spectroscopy
Benefits
- No ethical problem (non-invasive technique because skin exposure to UV is extremely low, with < 10 J/m²-eff or around 5% of 1 DEM),
- FPS, FPUVA and Critical Wavelength measurement in a single standard,
- Improved repeatability and reproducibility,
- Improvements in terms of time and costs,
- Elimination of technically difficult procedures (e.g. visual reading of DEM),
- A method conducive to continuous improvement.
YOUR STUDIES with WENEOS
- Analyses carried out in-house at our center of expertise from January 2025.
- HDRS results for a product are estimated within three weeks.
Clinical method
In vivo
PRINCIPLE
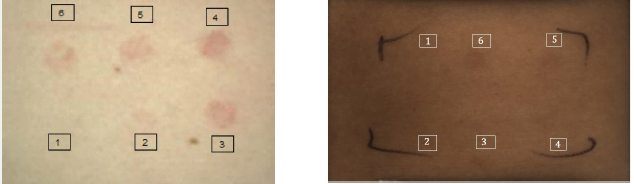
The in vivo method assesses the time it takes for a biological response to UV exposure to appear on the back of a human volunteer, by comparing areas with and without the application of a sunscreen product.
This assessment is based on two criteria:
- For UVB, by visually observing the Minimum Erythemal Dose (MED), corresponding to the first visible reddening of the skin.
- For UVA, by visually observing the Minimum Persistent Pigmentation Dose (MPPDD), which manifests itself as a lasting darkening of the skin.
Standards
- ISO 24444:2019: Cosmetics – Sun protection test methods – In vivo determination of sun protection factor (SPF)
- ISO 24442:2022: Cosmetics – Sun protection test methods – In vivo determination of UVA protection of a sunscreen product
- FDA 2021 : U.S. Food and Drug Administration – Final Administrative Order (OTC000006) – Over-the-Counter Monograph M020: Sunscreen Drug Products for Over-the-Counter Human Use
- ISO 16217:2020: Cosmetics – Sun protection test methods – Water immersion procedure for determining water resistance
- ISO 16217:2020: Cosmetics – Sun protection test methods – Percentage water resistance
Benefits
- Historical method,
- Compatible with all galenic formulations,
- Accepted in most countries.
YOUR STUDIES with WENEOS
- Analyses carried out externally by one of our partners.
- In vivo results for a product are estimated within 3-4 weeks.
Center excellence and expertise around sun protection
With over 25 years’ experience dedicated solely to the evaluation of sun protection, WENEOS is the largest center of expertise dedicated to the evaluation of sun protection, and offers various activities around this theme.
Sun protection evaluation laboratory
UVB | UVA | Blue Light | Visible | Infrared
PMMA substrate
HD6 Molded Plate | SB6 Sandlasted Plate
In vitro equipment
Robot SPREADMASTER | Chamber THERMASTER
Reference products
Solar products P2-P5-P8-S2 | HD0 Kit
Training
Theory | Practice | Technique